
Since the adoption of the Modernization of Cosmetics Regulation Act (MoCRA), cosmetic companies have been navigating a phased implementation of new regulatory obligations in the United States. As deadlines were introduced gradually, many organizations are now searching for clarity around one specific question: what is the MoCRA update in 2026?
Contrary to some assumptions, 2026 does not introduce a brand-new MoCRA regulation. Instead, it represents a transition from initial implementation to ongoing regulatory management. This article outlines what cosmetic companies should realistically expect in 2026, focusing on the key topics currently under discussion.
Why 2026 matters under MoCRA
MoCRA obligations did not enter into force all at once. Facility registration, product listing, adverse event reporting, and safety substantiation were introduced first, while other measures were intentionally delayed.
As a result, 2026 is often perceived as a “next phase” year. In reality, it marks a period where regulatory expectations become more operational, even when certain rules remain pending.
Fragrance allergens: what to expect in 2026
One of the most discussed MoCRA topics is the future U.S. regulation on fragrance allergens.
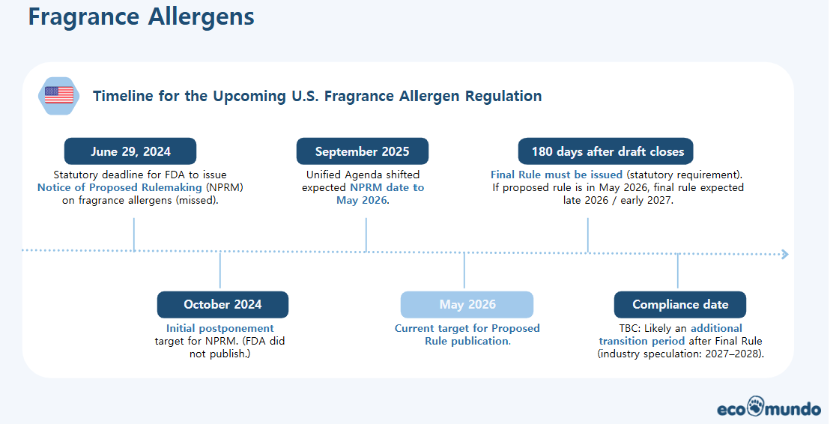
Under MoCRA, FDA is legally required to issue a rule establishing fragrance allergen disclosure requirements. However, the statutory deadline to publish the Notice of Proposed Rulemaking (NPRM) in June 2024 was missed. The timeline has since shifted multiple times.
Current regulatory planning indicates:
- the proposed rule is now targeted for May 2026,
- the Final Rule must be issued within 180 days after the draft closes,
- compliance is therefore expected late 2026 or early 2027, with a likely transition period.
Key takeaway:
2026 is not a compliance deadline for fragrance allergens, but it is a critical preparation year.
Good Manufacturing Practices (GMP): still pending, but not irrelevant
MoCRA requires FDA to establish mandatory cosmetic GMPs. However:
- the NPRM deadline in December 2024 was missed,
- the GMP rule was later classified as a “long-term action”,
- no draft rule is expected in the immediate future.
This means GMP requirements will not be finalized in 2026. Nevertheless, FDA already expects manufacturers to demonstrate structured manufacturing controls and product safety practices.
Facility registration renewal: a concrete 2026 obligation
Facility registration under MoCRA must be renewed every two years. This makes 2026 a critical year for companies that completed their initial registration in early 2024.

Key points to monitor:
- the first renewal cycle begins in early 2026,
- company information must remain accurate and complete,
- updates to brand names, Responsible Persons, and product category codes must be submitted,
- any change must be reported within 60 days.
For many companies, this will be the first real test of MoCRA data maintenance.
What cosmetic companies should focus on in 2026
Rather than preparing for new rules, companies should prioritize:
- maintaining accurate regulatory data,
- ensuring internal responsibilities are clearly defined,
- preparing for future allergen and GMP requirements,
- strengthening post-market surveillance and documentation readiness.
2026 is a year of regulatory consolidation, not regulatory disruption.
Conclusion
The MoCRA update in 2026 is not about reacting to new legal texts, but about operating within a more mature regulatory environment. Companies that focus on data accuracy, process ownership, and regulatory anticipation will be best positioned for the next phases of MoCRA implementation.
To help you go further on this topic, we’re making our recent webinar on North American cosmetic regulations available for free, on demand.
This session provides a practical overview of regulatory expectations for 2026, including MoCRA implementation timelines, compliance realities, and actionable insights you can apply directly in your organization.
👉 Watch the webinar replay:
https://form.ecomundo.eu/en/webinar-on-north-american-cosmetics-regulations-2026
Since the adoption of the Modernization of Cosmetics Regulation Act (MoCRA), cosmetic companies have been navigating a phased implementation of new regulatory obligations in the United States. As deadlines were introduced gradually, many organizations are now searching for clarity around one specific question: what is the MoCRA update in 2026?
Contrary to some assumptions, 2026 does not introduce a brand-new MoCRA regulation. Instead, it represents a transition from initial implementation to ongoing regulatory management. This article outlines what cosmetic companies should realistically expect in 2026, focusing on the key topics currently under discussion.
Why 2026 matters under MoCRA
MoCRA obligations did not enter into force all at once. Facility registration, product listing, adverse event reporting, and safety substantiation were introduced first, while other measures were intentionally delayed.
As a result, 2026 is often perceived as a “next phase” year. In reality, it marks a period where regulatory expectations become more operational, even when certain rules remain pending.
Fragrance allergens: what to expect in 2026
One of the most discussed MoCRA topics is the future U.S. regulation on fragrance allergens.

Under MoCRA, FDA is legally required to issue a rule establishing fragrance allergen disclosure requirements. However, the statutory deadline to publish the Notice of Proposed Rulemaking (NPRM) in June 2024 was missed. The timeline has since shifted multiple times.
Current regulatory planning indicates:
- the proposed rule is now targeted for May 2026,
- the Final Rule must be issued within 180 days after the draft closes,
- compliance is therefore expected late 2026 or early 2027, with a likely transition period.
Key takeaway:
2026 is not a compliance deadline for fragrance allergens, but it is a critical preparation year.
Good Manufacturing Practices (GMP): still pending, but not irrelevant
MoCRA requires FDA to establish mandatory cosmetic GMPs. However:
- the NPRM deadline in December 2024 was missed,
- the GMP rule was later classified as a “long-term action”,
- no draft rule is expected in the immediate future.
This means GMP requirements will not be finalized in 2026. Nevertheless, FDA already expects manufacturers to demonstrate structured manufacturing controls and product safety practices.
Facility registration renewal: a concrete 2026 obligation
Facility registration under MoCRA must be renewed every two years. This makes 2026 a critical year for companies that completed their initial registration in early 2024.

Key points to monitor:
- the first renewal cycle begins in early 2026,
- company information must remain accurate and complete,
- updates to brand names, Responsible Persons, and product category codes must be submitted,
- any change must be reported within 60 days.
For many companies, this will be the first real test of MoCRA data maintenance.
What cosmetic companies should focus on in 2026
Rather than preparing for new rules, companies should prioritize:
- maintaining accurate regulatory data,
- ensuring internal responsibilities are clearly defined,
- preparing for future allergen and GMP requirements,
- strengthening post-market surveillance and documentation readiness.
2026 is a year of regulatory consolidation, not regulatory disruption.
Conclusion
The MoCRA update in 2026 is not about reacting to new legal texts, but about operating within a more mature regulatory environment. Companies that focus on data accuracy, process ownership, and regulatory anticipation will be best positioned for the next phases of MoCRA implementation.
To help you go further on this topic, we’re making our recent webinar on North American cosmetic regulations available for free, on demand.
This session provides a practical overview of regulatory expectations for 2026, including MoCRA implementation timelines, compliance realities, and actionable insights you can apply directly in your organization.
👉 Watch the webinar replay:
https://form.ecomundo.eu/en/webinar-on-north-american-cosmetics-regulations-2026







